FRS Article

How One Strategic Discipline Will Help Your Drug Launch Succeed
by Kevin L. O'Neill
February 2020
The complexity and costs of launching a pharmaceutical product in today’s market have increased significantly over the last decade. The most recent paper from the Tufts Center for the Study of Drug Development puts the cost of developing a drug at $2.7 billion.1 It is well known that 90% of drug candidates fail to reach regulatory approval. Those products that clear the regulatory hurdle still face a high risk of commercial failure. A Bain and Company study reports that nearly 50% of recent drug launches failed to meet investor expectations.2 This confirms an earlier study by McKinsey reporting that 66% of pharma product launches were financial failures.3
Both drug and medical device companies acknowledge the uncertainty that they face launching their products into the market. A 2017 review of SEC filings found that every life science company (100%) cited their “ability to commercialize current and future products” as a major risk factor.4 Don’t let the gravity of that slip by: Every single life science company viewed commercializing their product in the post-regulatory approval period as a major risk factor.
So why would products that are approved for commercialization still fail? Don’t drug companies have complete control over their planning and preparations for a product launch? It seems reasonable to expect a higher success rate of launches than what is seen in the market.
Most product launch failures can be traced back to the fact that their preparation efforts do not support a satisfactory customer experience. When the market has not been suitably prepared to understand and receive the product or the product is inadequately prepared to resonate with the market, it tells us that the company failed to set a launch strategy that includes critical elements that the market needed or the product promotion should have supplied.
The common thread to successful launches is that the manufacturer has determined what conditions are necessary for their customers to have an optimal experience with their product, and then they do everything in their power to deliver on that experience. In the biopharmaceutical industry, this means having all the necessary components in place and integrated properly as part of a launch strategy to create what ForeRunner Strategy calls the Desired Customer Experience ™ (DCE).
In the life sciences, the Desired Customer Experience is realized when all necessary elements — value proposition, patient services, disease and drug awareness, supply, access, enabling technologies (diagnostics, genetic testing, etc.) and others — are in place that ensure the customer has the best possible experience in obtaining and using the product.
Bio-pharma companies devote considerable resources in preparing for a launch. But the launch teams often lose sight of the Desired Customer Experience. Recently, ForeRunner Strategy was invited to be part of a launch leadership team meeting with the goal of setting the vision for a successful launch. The product was two years from expected approval. All the key department heads were there: regulatory, clinical, medical affairs, supply, global marketing and others. The group was discussing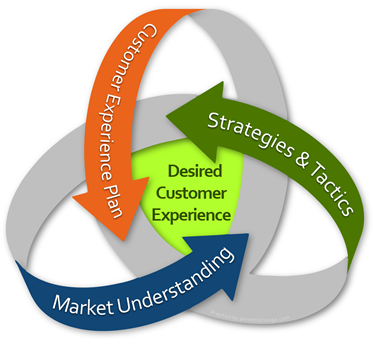 the status of building an integrated launch plan when the committee chair asked, “What do we mean by launch readiness?” The room fell silent, and then everyone spoke at once, voicing their divergent views on when they would be ready to launch. The regulatory affairs person said, “When the product has been approved and product labeling and promotional materials are released.” The supply person offered, “When launch quantities are released from the warehouse or received in the distribution center.” Market Access suggested, “When all key customers are briefed, formulary coverage is established, and product ordering systems are in place.” And the discussion continued until the committee chair finally said, “How can we reach consensus with so many differing opinions of launch readiness?” I replied, “Defining launch readiness is actually quite simple: Launch readiness is when you are able to deliver what we call the Desired Customer Experience ™. Launch readiness is the point in time when all of the key elements of the customer experience are in place. Those elements include anything that, in their absence, would disrupt the customer (patient or provider) from a positive experience in obtaining or using the product.” Delivering the Desired Customer Experience is that which ensures launch success.
the status of building an integrated launch plan when the committee chair asked, “What do we mean by launch readiness?” The room fell silent, and then everyone spoke at once, voicing their divergent views on when they would be ready to launch. The regulatory affairs person said, “When the product has been approved and product labeling and promotional materials are released.” The supply person offered, “When launch quantities are released from the warehouse or received in the distribution center.” Market Access suggested, “When all key customers are briefed, formulary coverage is established, and product ordering systems are in place.” And the discussion continued until the committee chair finally said, “How can we reach consensus with so many differing opinions of launch readiness?” I replied, “Defining launch readiness is actually quite simple: Launch readiness is when you are able to deliver what we call the Desired Customer Experience ™. Launch readiness is the point in time when all of the key elements of the customer experience are in place. Those elements include anything that, in their absence, would disrupt the customer (patient or provider) from a positive experience in obtaining or using the product.” Delivering the Desired Customer Experience is that which ensures launch success.
The concept of a Desired Customer Experience is a simple one. Although developing a DCE is not easy, it brings great value to the launch team, company leadership, and eventually the customer. It creates a vision and ownership for all those involved in preparing for the product launch. For the customer, it creates a positive experience and a high level of satisfaction with the product.
During the process of planning for market entry, launch teams must create a clear vision of the Desired Customer Experience, define which elements support the vision, translate those into launch imperatives, and finally incorporate those imperatives into an integrated launch plan. Launch timing is dependent on all of the elements of the DCE, not simply product availability or sales force readiness. This is especially true with the newer technologies like cell and gene therapies that involve complex customer interactions.
The value of the integrated launch plan should not be underestimated, since it is the operating plan which delivers the Desired Customer Experience.
All the elements needed to support the DCE for a particular product are “launch critical.” Therefore, launch readiness is defined as the point in time when all the key elements of the Desired Customer Experience are in place to ensure the customer has the best possible experience in obtaining and using the product. The development of the DCE is a key component in the pre-launch process. Creation of the DCE should be timed appropriately to inform the subsequent development of an integrated launch plan.
ForeRunner Strategy brings to its clients the acuity needed to ask key questions which yield critical thinking about the launch strategy and plans. We combine deep expertise with disciplined and proprietary planning approaches, and the ability to derive actionable insights that help our clients launch products successfully.
1 DiMasi, JA, Grabowski, HG, Hansen, RW, “Innovation in the pharmaceutical industry: New estimates of R&D costs”, J of Health Economics 47, (May 2016), 20-33.
2 “How to Make Your Drug Launch a Success,” Bain & Company 2017
3 “The secret of successful launches” McKinsey & Company 2014
4 BDO Life Sciences Risk Factor Report, Binder Dijker Otte, 2017
* graphics by presentationgo.com



